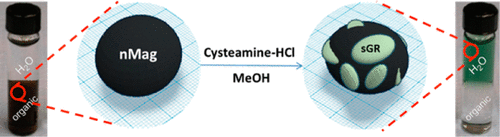
A novel, reactive green iron sulfide (sulfide green rust) formed on iron oxide nanocrystals
C. Jones, S. Chattopadhyay, N. I. Gonzalez-Pech, C. Avendano, N. Hwang, S. S. Lee, M. Cho, A. Ozarowski, A. Prakash, J.T. Mayo, C. Yavuz, V. L. Colvin
Chem. Mater., 27 (3), 700-707
2015