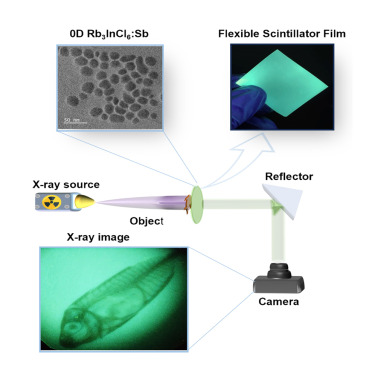
Lead-free, all-inorganic halide nanocrystals hold promise for advanced devices, with chloride-based variants offering stability, tunable band gaps, and easy processing. However, these nanocrystals face synthesis challenges due to the low solubility and complex coordination of heavy metal chloride salts. The conventional ligand-assisted reprecipitation (LARP) method is incompatible with chloride-based crystals, while the hot-injection technique is slow and complex, hindering efficient synthesis and scalability. We present a modified LARP method using an acid-mediated strategy to synthesize undoped and Sb3+-doped Rb3InCl6 nanocrystals. These nanocrystals maintain colloidal stability for up to 6 months and exhibit a large Stokes shift (230 nm), a high photoluminescence quantum yield (PLQY) of 68.01%, low self-absorption, and a high light yield of 15,500 photons/MeV. The flexible nanocrystal composite film with polysulfone achieves an X-ray imaging resolution of 18.5 line pairs per millimeter (lp mm−1). The colloidal solution and film of Rb3InCl6:Sb nanocrystals exhibit strong radioluminescence and linear responses under X-ray irradiation, highlighting their potential for medical radiography.