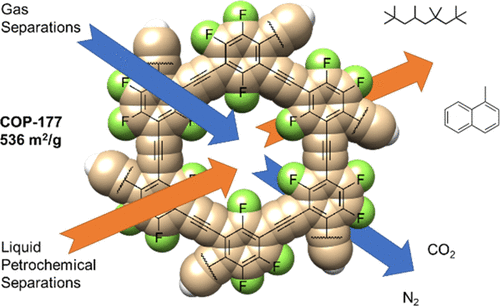
Nanoporous polymers offer great promise in chemical capture and separations because of their versatility, scalability, and robust nature. Here, we report a general methodology for one-pot, metal-free, and room-temperature synthesis of nanoporous polymers by highly stable carbon–carbon bond formation. Three new polymers, namely, COP-177, COP-178, and COP-179, are derived from widely available perfluoroarenes and found to be superhydrophobic, microporous, and highly stable against heat, acid, base, and organic solvents. Nitrile, amine, and ether functionalities were successfully installed by SNAr-type postfunctionalization and were shown to increase CO2 uptake twice and CO2/N2 selectivity 4-fold. Due to its inherent superhydrophobicity, COP-177 showed high organic solvent uptake both in liquid and vapor form. Furthermore, in a first of its kind, by combining microporosity and hydrophobicity, COP-177 separated two small molecules with the same boiling point in a continuous column setting.