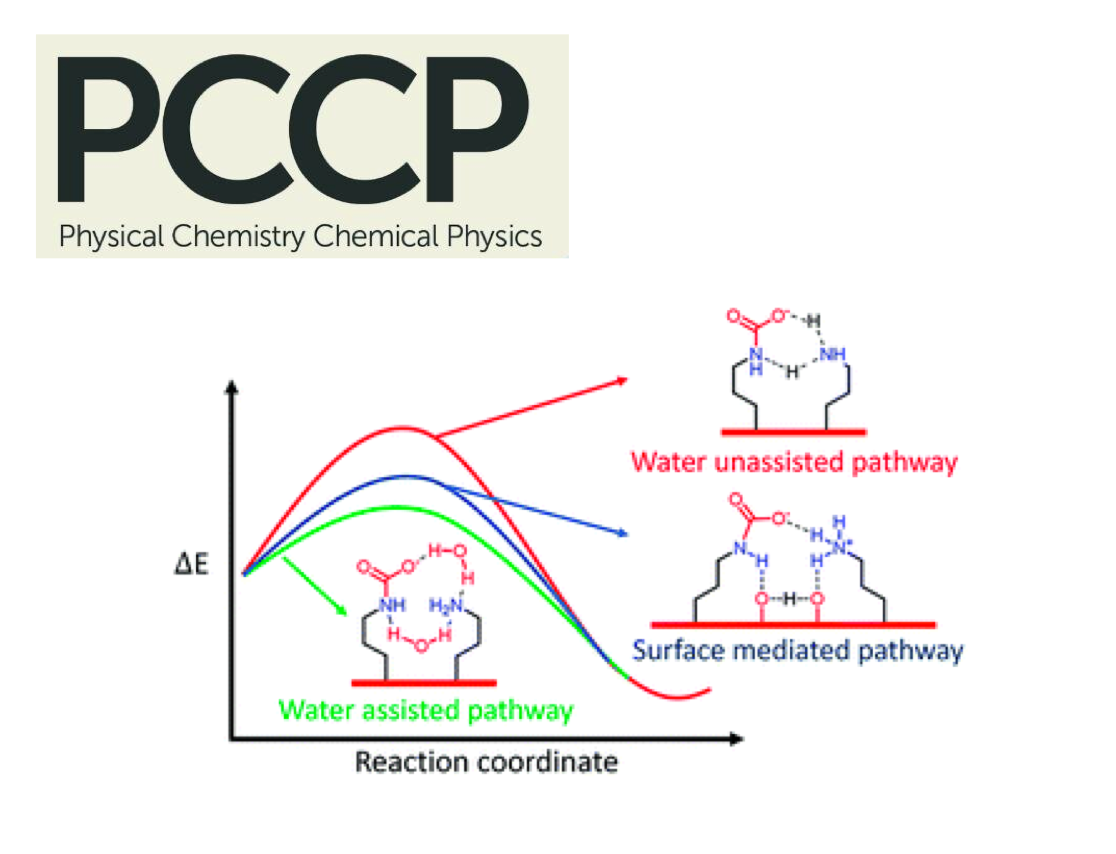
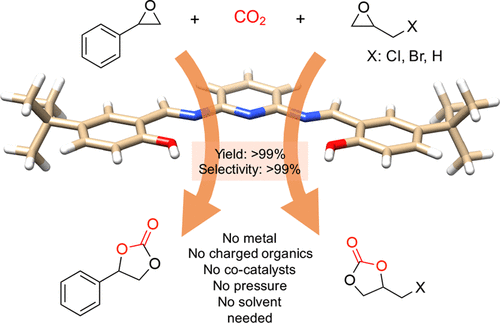
Cyclic carbonates as industrial commodities offer a viable nonredox carbon dioxide fixation, and suitable heterogeneous catalysts are vital for their widespread implementation. Here, we report a highly efficient heterogeneous catalyst for CO2 addition to epoxides based on a newly identified active catalytic pocket consisting of pyridine, imine, and phenol moieties. The polymeric, metal-free catalyst derived from this active site converts less-reactive styrene oxide under atmospheric pressure in quantitative yield and selectivity to the corresponding carbonate. The catalyst does not need additives, solvents, metals, or co-catalysts, can be reused at least 10 cycles without the loss of activity, and scaled up easily to a kilogram scale. Density functional theory calculations reveal that the nucleophilicity of pyridine base gets stronger due to the conjugated imines and H-bonding from phenol accelerates the reaction forward by stabilizing the intermediate.