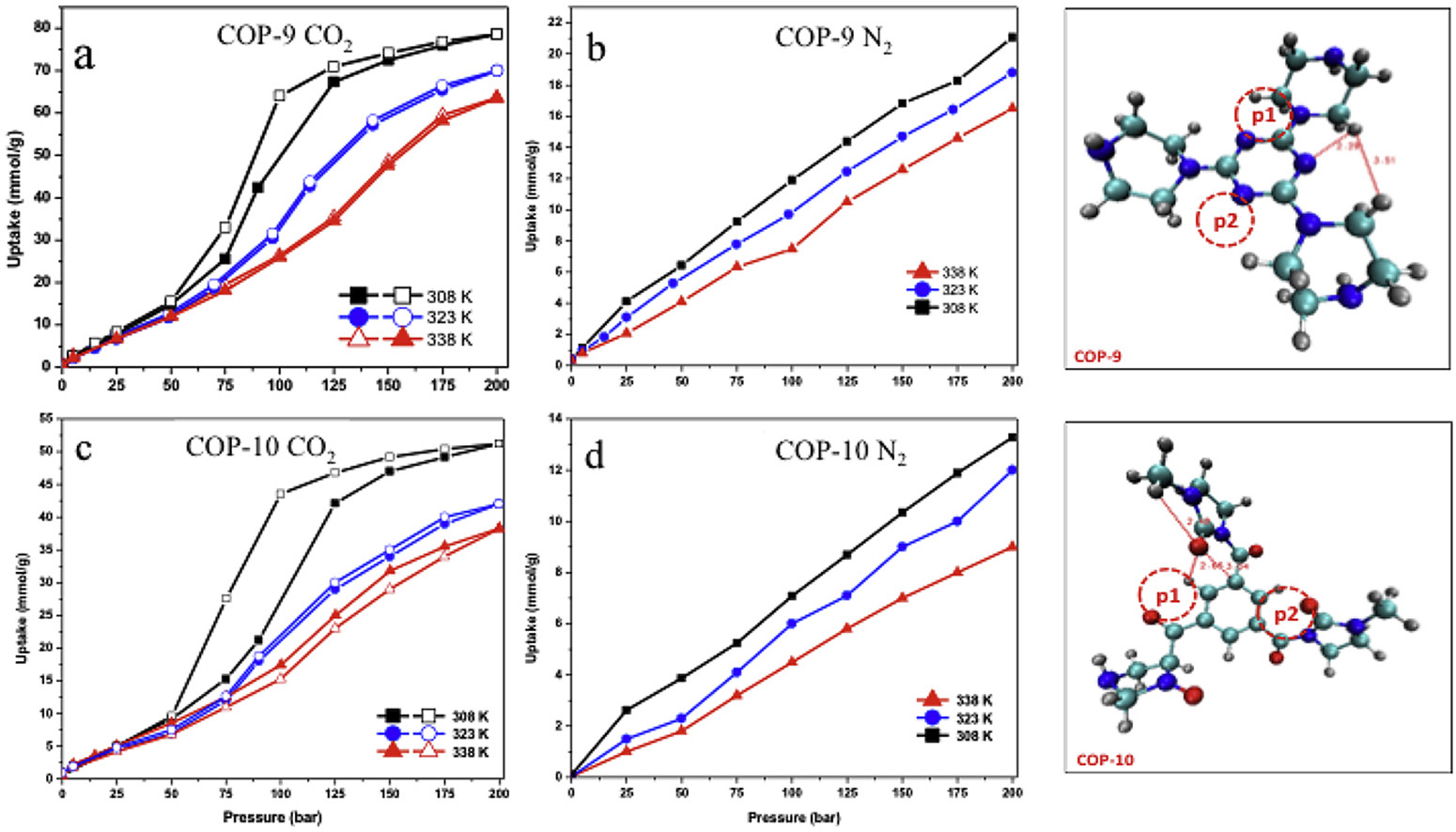
In this manuscript, we report synthesis, characterization and application of amine and amide type covalent organic frameworks as CO2 adsorbent materials at various isotherms and wide pressure conditions. Furthermore, we also report a detailed density functional theory investigation of the studied adsorbents in order to explain their adsorption behaviors and provide comparisons with experimental results. The objective of this work was to investigate custom design porous polymers by building amine and amide functionalities in the final structures, whether they have efficient CO2 capturing performances at wide process conditions that covers both low and high pressure end applications to cover either pre- or post-combustion processes. On the other hand, energy storage performances of these materials were tested by performing H2 sorption experiments as well. Two porous polymers, namely COP-9 and COP-10, were characterized with BET, TGA and FTIR to evaluate the physical properties of studied porous polymers and then were tested for CO2, N2 and H2 adsorption both at low and high pressures. Studied materials were found to have compelling adsorption capacity mostly at high pressures and have very good selectivity for CO2/N2 and CO2/H2 respectively.