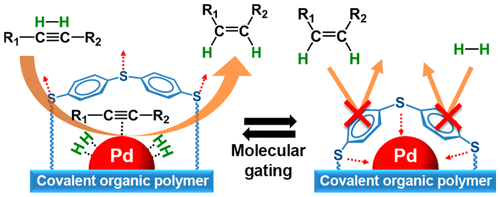
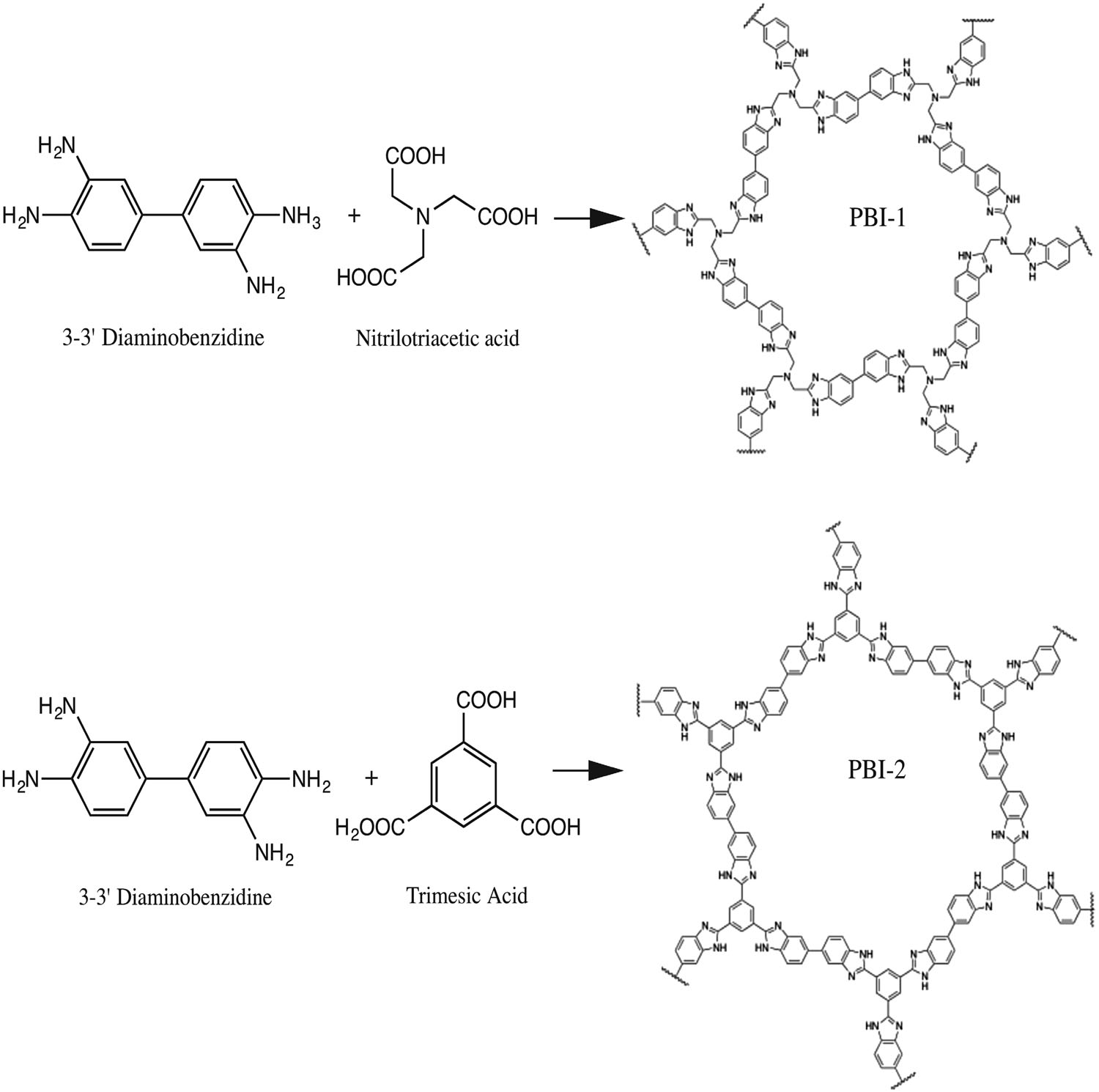
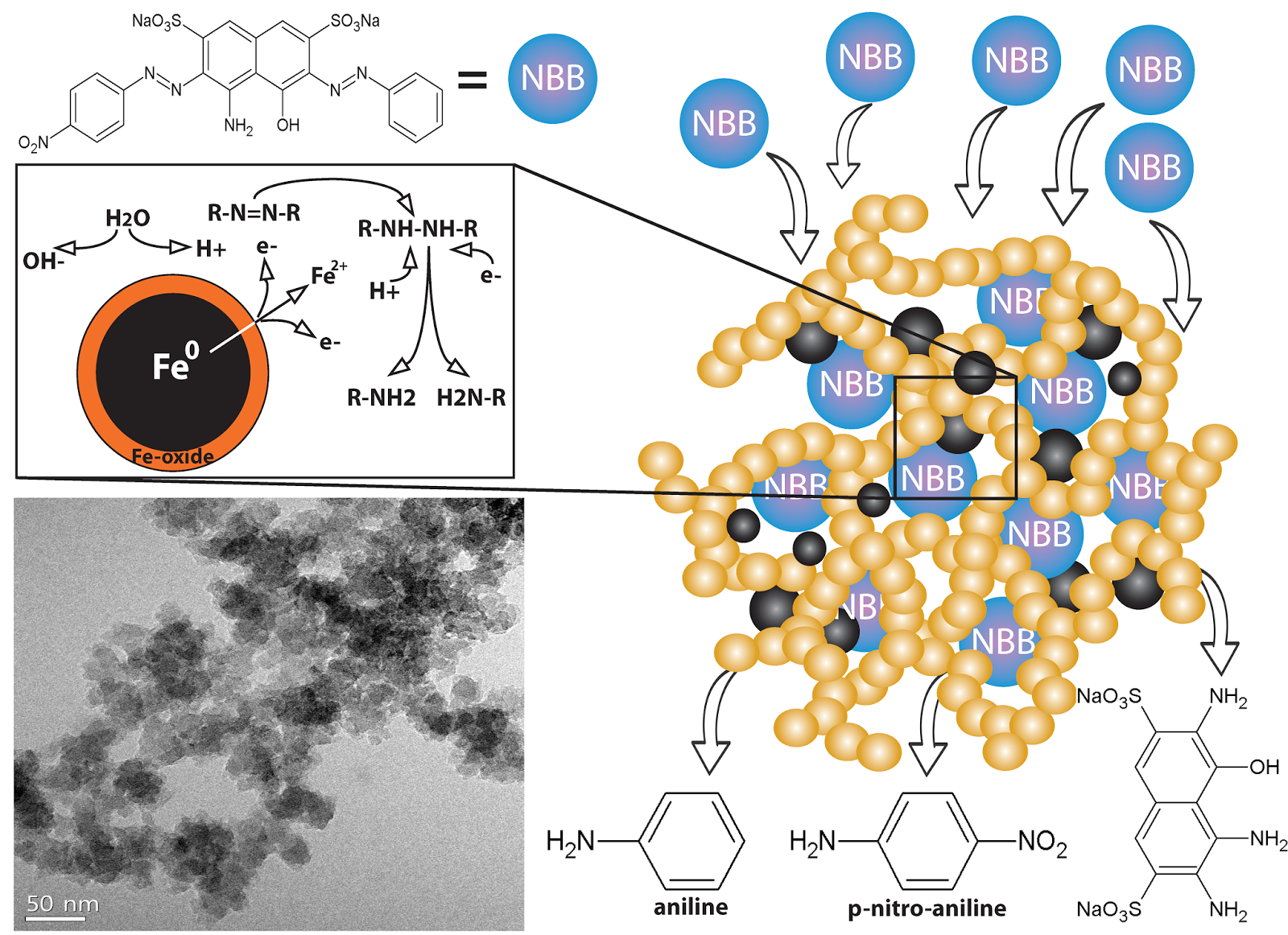
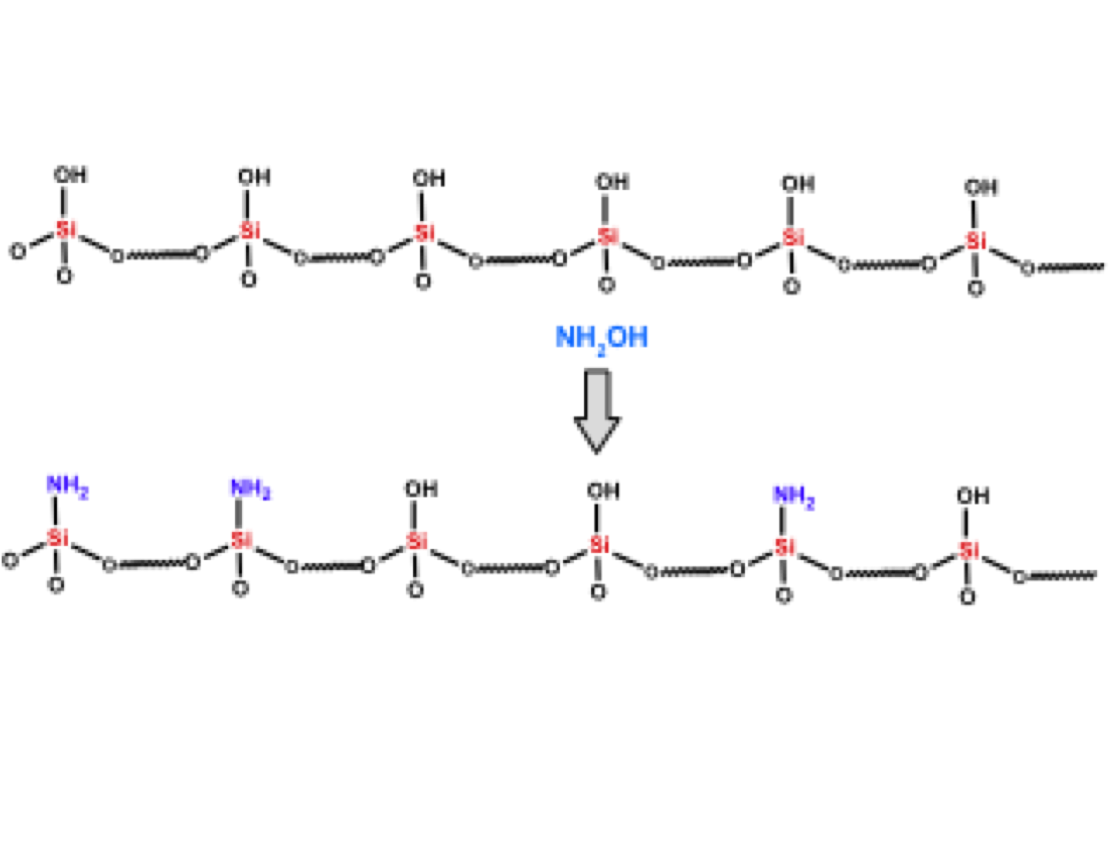
A new carbon–carbon bonded nanoporous polymer network was synthesized via efficient and catalyst free Knoevenagel-like condensation polymerization in near quantitative yields. The obtained polymer network, Covalent Organic Polymer – COP-100 possesses strong fluorescent properties and designed solubility in polar aprotic solvents, which shows promise for use as a metal-sensing material in solution. COP-100 exhibited high selectivity towards Fe2+ and Fe3+ in the presence of other common metal cations (Al3+, Ag+, Cd2+, Co2+, Cr3+, Cu2+, Hg2+, Mg2+, Mn2+, Na+, Ni2+, Zn2+) as the fluorescence of the polymer was significantly quenched even at very low concentrations. In the range from 2.5 × 10−6 to 2 × 10−4 M, a linear fluorescence emission response with equipment limited detection minimum of 2.13 × 10−7 M and 2.45 × 10−7 M for Fe2+ and Fe3+, respectively, was observed. These results suggest that COP-100 is a promising material as a selective fluorescence sensor for iron ions.